Automating the creation of dose escalation safety summary reports for Phase I trials
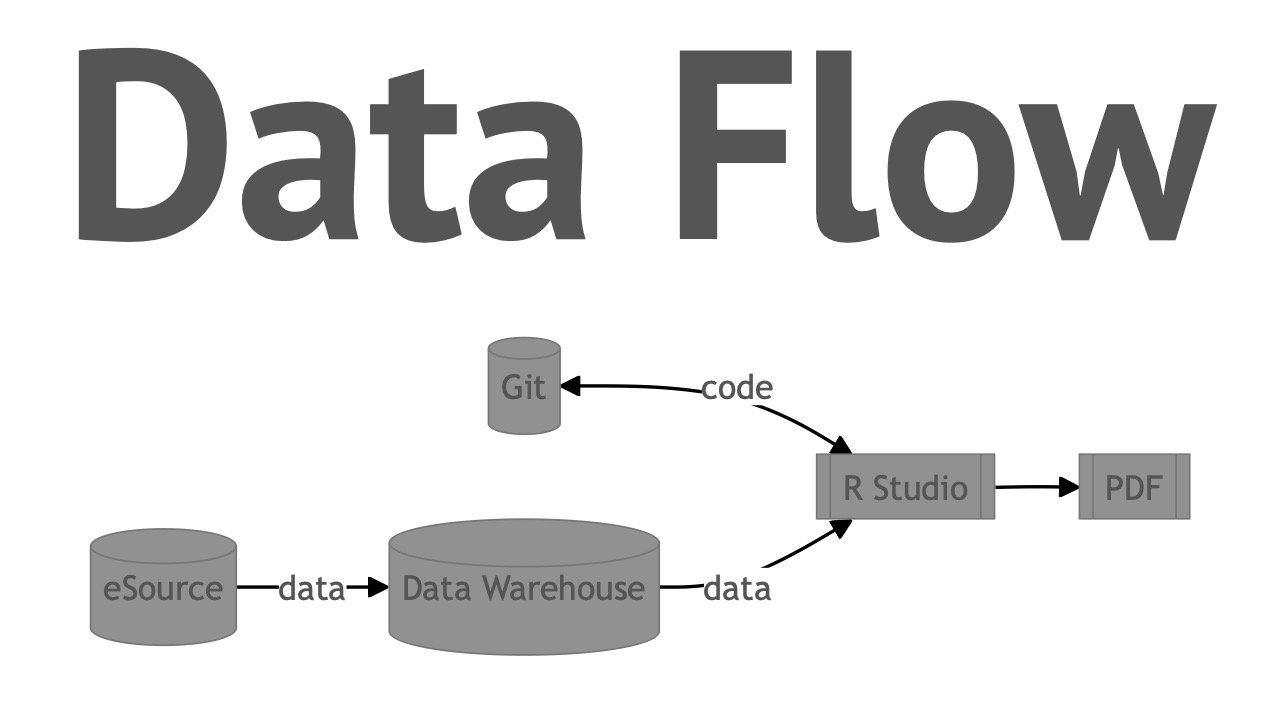
From eSource right through to Safety Summary Report
Introduction
I have reworked an old presentation that I sweated over profusely at the time, staying up late and sober to finish it off, whilst my colleagues were having one last drink.
It made sense to me, but I am not sure it made sense to anybody else.
I think I struggled, not being a data scientist, but being familiar with the problem space.
Things have moved on surprisingly quickly since that day in Chicago in 2017 with analytical tools such as as R, Python and Jupyter becoming trusted and more prevalent.
The newer open source Quarto project seems particularly interesting compared to R Markdown / R Studio, as it is mulitlingual.
So I have had another go and have tried to strip it down to the basics.
Did I pare it down too much?
Does it make any more sense now?
Does your Phase I Unit do this already? Am I now soooo out of touch? 😄
If so, tell me all about it!
Links from the report
- Accelerating the Production of Safety Summary and Clinical Safety Reports - a Model to Aspire to. Steffan Stringer, 2017
- Clinical Reporting With R, Revolution Analytics, 2010
- Markdown - Wikipedia
- R
- Git - Wikipedia
- Janssen’s R service for visualization and processing
- Open Source Statistical Software (OS3) in Pharma Development: A case study with R, Anthony Rossini, David A. James, presented at useR!, 2007
- Using R: Perspectives of a FDA Statistical RevieweR, Mat Soukup, presented at useR!, 2007
- R, drug development and the FDA, Joseph Rickert, Revolutions, 2013
- Statistics Using R with Biological Examples, Kim Seefeld & Ernst Linder, The Comprehensive R Archive Network, 2007
- R for Clinical Trial Reporting: Reproducible Research, Quality and Validation, Frank Harrell, useR! 2007 Conference, 2007
- Example Closed Meeting Data Monitoring Committee Report, Frank Harrell, 2007
- R: Regulatory Compliance and Validation Issues A Guidance Document for the Use of R in Regulated Clinical Trial Environments, The R Foundation for Statistical Computing, 2014
- R Markdown - from R Studio
- R Markdown: The Definitive Guide
- A better way to deploy R & Python
- sweave
- knitr
- rreport
- Example Closed Meeting Data Monitoring Committee Report, FE Harrell, 2020
- DSMB Report for EXAMPLE Trial, FE Harrell, 2020
- R for Graphical Clinical Trial Reporting, Frank Harrell, 2020
- R For Graphical Clinical Trial Reporting, Frank Harrell, Regeneron, 2022
- Welcome to Quarto - An open-source scientific and technical publishing system