
Automating the creation of dose escalation safety summary reports for Phase I trials
I have reworked an old presentation that I sweated over profusely at the time, staying up late and sober to finish it off, whilst my colleagues were having one last drink.
It made sense to me, but I am not sure it made sense to anybody else.
I think I struggled, not being a data scientist, but being familiar with the problem space.
Things have moved on surprisingly quickly since that day in Chicago in 2017 with analytical tools such as as R, Python and Jupyter becoming trusted and more prevalent.
So I have had another go and have tried to strip it down to the basics.

Ready for an eSource and automation solution at your Clinical Pharmacology Research Unit?
I am a huge fan of eSource & automation software in Early Phase (‘Phase I’) trial settings and have been working with them for more than 20 years now.
In early articles I have written about eSource Stakeholders, What is eSource and how is it different to EDC?, and eSource Systems - what’s included?.
I do have a bias in that almost all my experience has been working in, or with, Contract Research Organisation (CRO) clinical pharmacology research units conducting commercially sponsored clinical trials.
I am not trying to put smaller, academic sites off from going paperless - you should!
As fast as you can!

eSource Stakeholders
As usual I am focusing on users of eSource and automation systems in ‘Clinical Pharmacology Research Units’ (CPRUs, CPUs, Phase I Units, Clinical Trial Units etc.).
I am splitting the users in two ways - site versus sponsor, and by the immediacy of the benefit, their ‘proximity’ to the application, whether they are using the software everyday - primary, secondary and tertiary stakeholders.
The roles are based on my personal experience of working in four different CPRUs and supporting many customers implementing eSource solutions. I appreciate not all sites will have all these roles, and if they do, might name them differently.
Feel free to let me know if I missed out an obvious stakeholder group or whether you’d put them at a different level in the hierarchy.
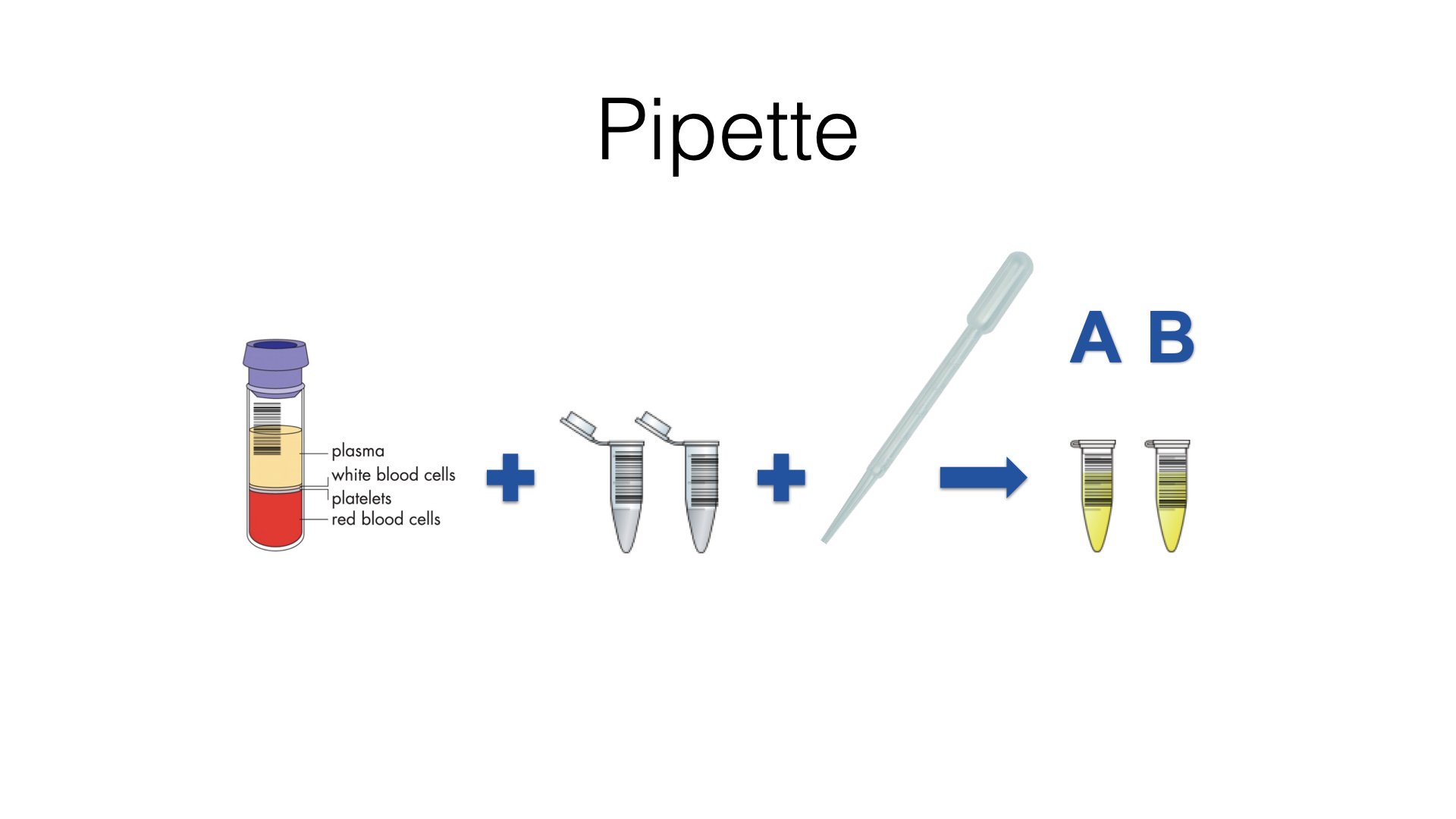
Can your early phase eSource and automation software handle this very simple workflow?
This is not rocket science, but I am hearing that software solutions touted as being suitable for use in early phase clinical pharmacology research settings, can’t support this basic flow utilising barcoded sample tubes.
What is eSource and how is it different to EDC?
My focus here is all about eSource tools and systems that involve automation (think barcoding, tasks etc.) and integration of medical devices in a Clinical Pharmacology Research Unit (Phase I / healthy volunteer) setting. Would love to have your feedback on what I missed and any questions you might have, and I will do my best to help.

Eating your own dog food
I have worked in a number of small to medium size teams, some scrappy startups and others embedded in monolithic corporate entities. In latter years have focused on driving the use of ‘e’ technologies in clinical research.
For a small team of engineers, testers and product specialists to be successful, to punch above its weight, I strongly believe they need to eat their own dog food (it’s a reference).

eSource Systems - what’s included?
eSource systems for early phase clinical trials have been around for a long time. My first direct exposure was over 20 years ago when working in a well known London Phase I site. The software had already been successfully deployed and was in widespread use at its sister unit in Berlin.
These systems have to be good at addressing the core needs of their users - being really good at capturing clinical trial data in real time at the bedside. They must be secure, they must be reliable and they must support and comply with GxP and other applicable rules and regulations.
The best of these systems will be standards-based (e.g. CDISC ODM) integrate with local safety labs and common medical devices and use automation to support efficiency and compliance.

eCOA in eSource and early phase trials
Apologies in advance, as I am writing this essay from a position of inexperience and ignorance. My eHealth experience is heavily biased to early phase / clinical pharmacology trials. Rating scales are used quite often and participant diaries are used occasionally. Where I used to see these, they were still pen and paper based or using third party electronic tools from the big vendors and supplied via the sponsor. They were never integrated with or part of the site’s eSource platform.
Having said that I wanted to sketch out some design ideas that have been influenced and informed by my own recent unexpected visits to urgent care in the US and A&E (accident and emergency) in the UK, and multiple follow-up visits since.

Careers in Life Sciences
I helped a friend out this week by manning a school careers fair stand representing Blackwater Sciences. Somewhat counter to the stated mission, I slipped in some subliminal messaging that work is not everything.
My rolling slide deck included the 'three buckets' (it might have been baskets in the original!) philosophy of work / life balance. You have three buckets - your work, your hobbies and your home / family life. If you can keep any two of these buckets full, the chances are you will be happy and fulfilled.

